Blood is central to the great advances in healthcare that have happened in the last century. More than 110 million blood transfusions are performed each year for a range of medical procedures, whether it is to treat anemia, sickle cell diseases, severe bleeding, or in cancer treatments. Blood components are also key to many treatments, for instance bleeding disorders such as haemophilia which can be treated with plasma-derived medicines. In medical research, blood serve a range of useful purposes, for instance to study blood-borne diseases.
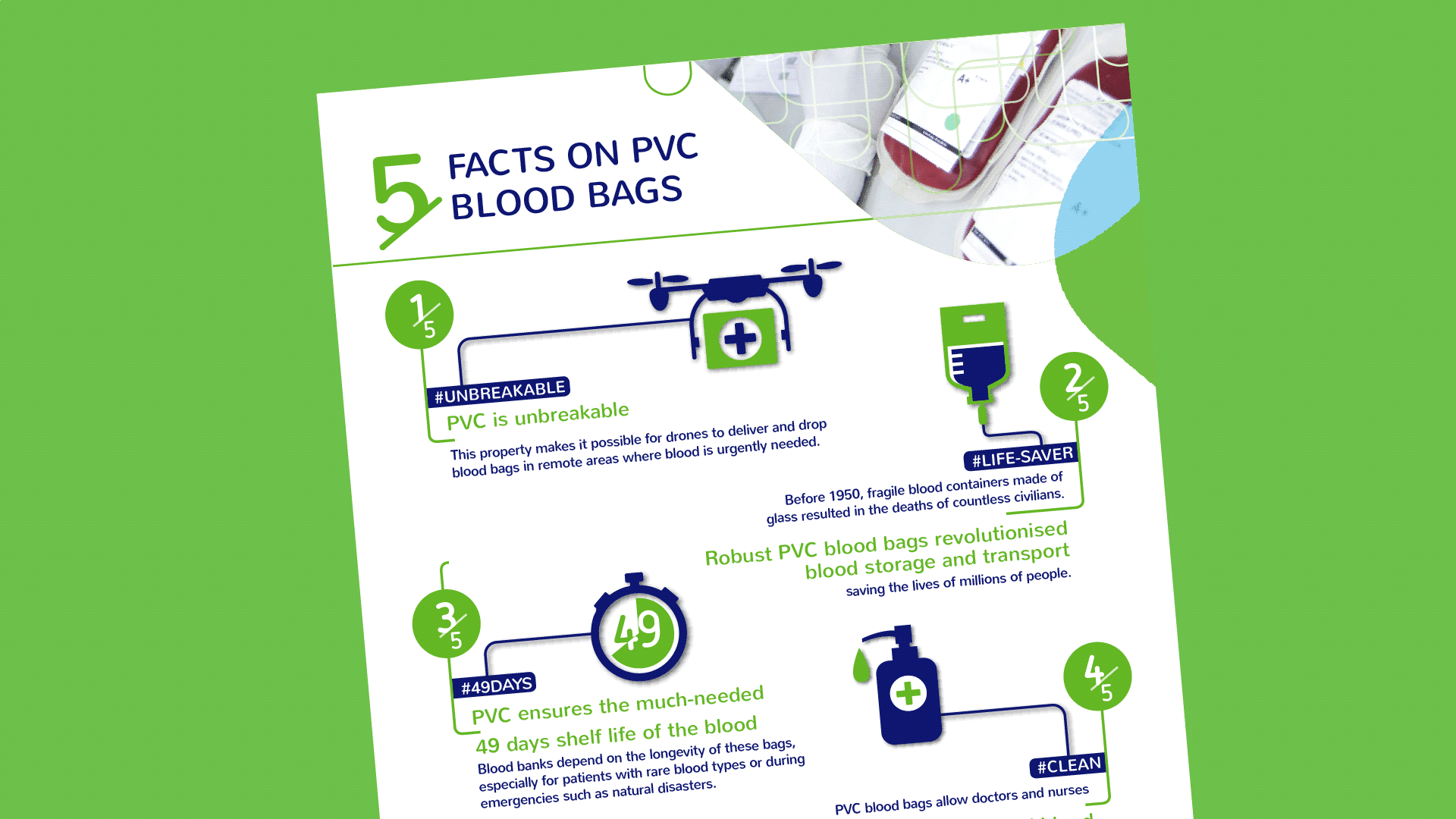
All this could not be made possible without the many million donors around the world and the fine-meshed network of blood banks. Since the 1950s, blood systems have depended on the polyvinyl chloride plastic bag, which provide a remarkable storage time of up to 49 days. Now, the next generation PVC blood bags are imminent.
Why is a storage period for up to 49 days necessary?
PVC blood bags allow a long shelf life for the blood. Under refrigeration, the blood can last for up to 49 days. The minimum requirement from European blood banks is 42 days. While much blood is used within a few weeks, there are several reasons why a storage period of up to 49 days is crucial. It is essential that any attempts to develop blood bags with alternative materials take these considerations into account.
The future of blood storage: Progress towards non-DEHP blood bags
As foundational material in the context of blood bags used to collect, process, and store the whole blood and blood components, PVC is currently undergoing a significant transformation. Historically, the PVC components of a blood collection system (collection bags, plasma bags, red blood cell bags, tubing parts, connector parts) have relied on DEHP (di(2-ethylhexyl) phthalate) as a plasticiser. This substance has specifically played a key role in preserving the stability and integrity of red blood cells (RBCs) during storage, which is essential for effective blood transfusions.
The evolving regulatory landscape, particularly within the European Union, reflects mounting concerns about the potential toxicity of DEHP. The updated EU Medical Device Regulation and the REACH regulation now mandate the phase-out of DEHP from blood bags before 1 July 2030. This development marks a crucial juncture in healthcare, underscoring the pressing need for alternative plasticisers that can match DEHP's performance while maintaining the quality of RBCs.
In response to this imperative, extensive research efforts have been directed towards identifying suitable substitutes for DEHP. Alternative non-phthalate plasticisers such as DINCH (1,2-Cyclohexanedicarboxylic acid, diisononyl ester), DEHT (di(2-ethylhexyl) terephthalate), and BTHC (n-butyryl tri-n-hexyl citrate) are currently under exploration.
Importantly, these potential substitutes have undergone rigorous testing in compliance with REACH and the EU Medical Device Regulation, confirming their safety and efficacy. They are also listed as alternatives to DEHP in the European Pharmacopeia, which sets the standard for medicines and medical devices in Europe and beyond. Notably, these substitutes have already, to a large degree, replaced DEHP in life-saving medical devices and other healthcare applications where PVC is used.
PVCMed partner RENOLIT leads the way to non-DEHP blood bags
The transition presents formidable challenges, as it may entail not only changing the plasticiser but also modifying the red blood cell storage solution to uphold the necessary quality of RBCs. The process involves comprehensive validation efforts, as outlined in the recent guidelines developed by members of the European Blood Alliance that call for a 3-phase standardised approach to evaluate blood components collected, prepared, and stored in non-DEHP devices.
Despite these challenges, the results of these tests show promising outcomes. RENOLIT Healthcare, a valued partner of the PVCMed Alliance and a leading film producer for blood bags and other medical applications, is at the forefront of this transition. Early test results of blood bags using a prototype non-phthalate PVC film, called RENOLIT Bloodprotect 42Plus, show RBC haemolysis level (0.17 ± 0.1 %) with no significant difference to DEHP (0.16 ± 0.2 %) after 42 days of storage, when using conventional SAG-M storage solution. The results from the Institute of Hematology and Transfusion Medicine, Warsaw, Poland, have been presented at the ISBT Gothenburg Congress in June 2023 and are published in the leading scientific journal on blood transfusion Vox Sanguinis.
Promising results with other solutions were also presented at the congress: By changing to PAGGS-M solution, Dutch national blood bank Sanquin has found hemolysis rates comparable to DEHP for red blood concentrate (RCC) collected in DINCH plasticised collection bags and stored for 42 days in blood bags plasticised with DINCH, DEHT, or BTHC. The French Blood Establishment (EFS) has found that RCCs stored in a PVC/Citrate/PAGGS-M storage bag for 49 days were compliant with French quality requirements, as were plasma prepared and stored in a DINCH plasticised PVC container.
The progress made by RENOLIT Healthcare and others in developing non-DEHP solutions for blood bags is a potential game-changing development in the field of medical storage. However, it is important to note that this is a long-term endeavour with a horizon extending beyond five years.
RENOLIT Healthcare's pivotal role in this effort aligns seamlessly with PVCMed Alliance's dedication to the safe and sustainable use of PVC in healthcare.
Did you know robust plastic replaced breakable glass?
PVC is the material of choice for blood bags and has been so for over 60 years. In 1950, two American doctors invented a plastic bag for blood. Prior to that, fragile glass containers were used. The new robust plastic bag enabled a revolution in blood collection and preparation. Now, safe and easy preparation of multiple blood components from a single unit of whole blood was made possible. The plastic bag could simply withstand the high g-force it is exposed to when blood is separated into plasma, red blood cells and platelet concentrates in the refrigerated centrifuge.
The new plastic blood bag first showed its worth during the Korean War. As it could withstand being dropped from the air without shattering it helped save thousands of soldiers’ lives. Robustness continues to be a key advantage. In various parts of Africa, drones deliver blood much faster than would be possible with land transport. Instead of a 5-hour round-trip drive to a hospital, average time for a drone delivery is 30 minutes.